A) I
B) II
C) III
D) IV
E) V
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is antiaromatic? 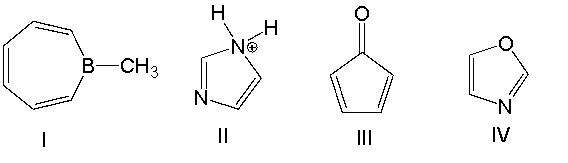
A) I
B) II
C) III
D) IV
E) none of these
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the common name for o-xylene.
A) hydroxybenzene
B) aminobenzene
C) 1,2-dimethylbenzene
D) ethylbenzene
E) 1,3-dimethylbenzene
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the structures of the intermediates and final product in the following reaction sequence. 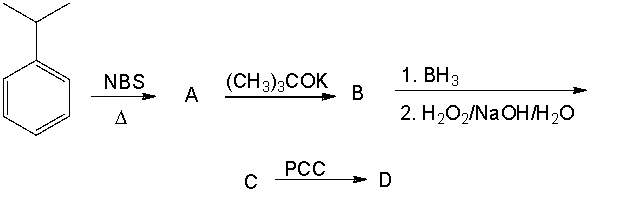
Correct Answer

verified
Correct Answer
verified
Essay
Using a Frost circle, draw the molecular orbital energy diagram for the cyclopropenyl anion and predict if it is aromatic. 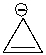
Correct Answer

verified
 _TB4454_00...
_TB4454_00...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Using a Frost circle, draw the molecular orbital energy diagram for the cyclopentadienyl anion and predict if it is aromatic. 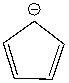
Correct Answer

verified
 _TB4454_00...
_TB4454_00...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the main difference between an aromatic and antiaromatic compound?
A) Aromatic compounds must be cyclic and planar, but not antiaromatic compounds
B) Aromatic compounds must be monocyclic.
C) Antiaromatic compounds must have a conjugated system with a p orbital at every vertex
D) Aromatic compounds must satisfy Hückel's rule.
E) none of these
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to molecular orbital theory, how many -bonding molecular orbitals does benzene have?
A) 1
B) 2
C) 3
D) 4
E) 5
G) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Predict the product for the following reaction. 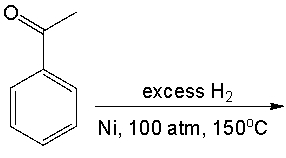
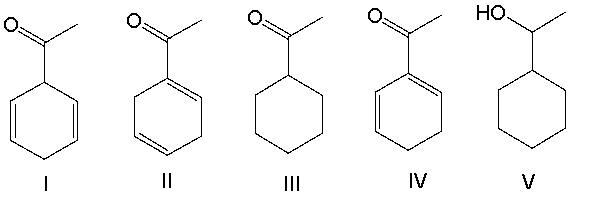
A) I
B) II
C) III
D) IV
E) V
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Provide the reagent(s) necessary to convert toluene to benzoic acid.
A) Na2Cr2O7/H2SO4/H2O
B) 1. NBS, 2.NaOH
C) 1. LiAlH4 2. H3O+
D) H2, Pd
E) 1. CO2, 2. H3O+
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
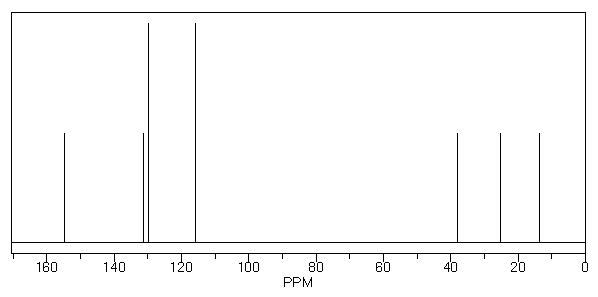
A compound with molecular formula C9H12O displays the following 1H NMR and 13C NMR spectra. Propose a structure for this compound. 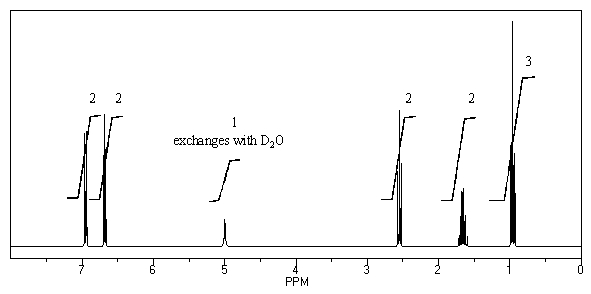
Correct Answer

verified
11ea894b_ad1e_5546_bba7_71a98916e37c_TB4454_00_TB4454_00
Correct Answer
verified
Essay
Both pyridine and pyrrole are nitrogen containing aromatic heterocyclic compounds. When treated with HCl, only pyridine forms the hydrochloride salt, whereas pyrrole is unreactive. Provide an explanation for this observed reactivity. 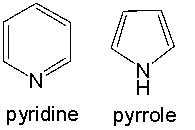
Correct Answer

verified
In pyridine, the nonbonding electron pai...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which one of the following compounds is most acidic? 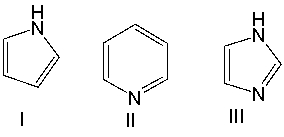
A) I
B) II
C) III
D) I & III
E) II & III
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is most acidic? 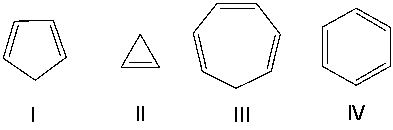
A) I
B) II
C) III
D) IV
E) none of these
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is aromatic? 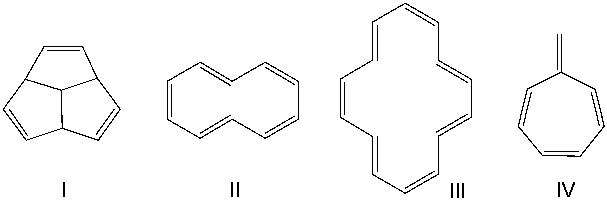
A) I
B) II
C) III
D) IV
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the functional group in styrene.
A) ether
B) alkene
C) carboxylic acid
D) aldehyde
E) ketone
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the reagents necessary to carry out the following conversion. 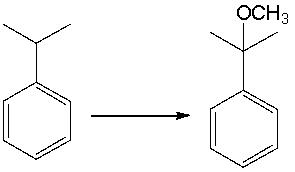
Correct Answer

verified
1. NBS/
2....View Answer
Show Answer
Correct Answer
verified
2....
View Answer
Multiple Choice
Which one of the following compounds is aromatic? 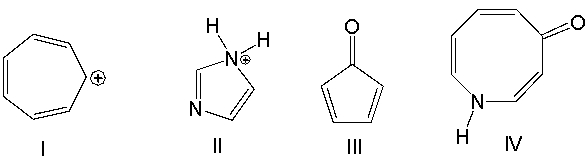
A) I
B) II
C) III
D) IV
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the common name for the following compound? 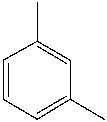
Correct Answer

verified
m-xylene
Correct Answer
verified
Essay
Using a Frost circle, draw the molecular orbital energy diagram for the tropylium cation and predict if it is aromatic. 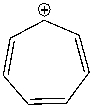
Correct Answer

verified
 _TB4454_00...
_TB4454_00...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 1 - 20 of 118
Related Exams