Correct Answer

verified
Correct Answer
verified
Multiple Choice
At a certain temperature,Kc equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g) . If a 2.50-L flask contains 0.400 mol of CO2 and 0.100 mol of O2 at equilibrium,how many moles of CO are also present in the flask?
A) 0) 422 mol
B) 0) 169 mol
C) 0) 107 mol
D) 0) 0114 mol
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is true for a reaction with Kc equal to 2.43 × 10-12?
A) Increasing the temperature will not change the value of Kc.
B) There are appreciable concentrations of both reactants and products.
C) The reaction proceeds hardly at all towards completion.
D) The reaction proceeds nearly all the way to completion.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent the initial state and the equilibrium state for the gaseous state reaction of A2 molecules (shaded spheres) with B atoms (unshaded spheres) to give AB molecules. 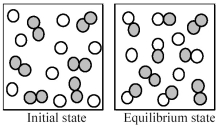 -If the volume of the equilibrium mixture is decreased,what will happen to the number of AB molecules and the number of B atoms?
-If the volume of the equilibrium mixture is decreased,what will happen to the number of AB molecules and the number of B atoms?
A) The number of AB molecules and the number of B atoms will both decrease.
B) The number of AB molecules will increase;the number of B atoms will decrease.
C) The number of AB molecules will decrease;the number of B atoms will increase.
D) The number of AB molecules and the number of B atoms will both increase.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Kc is 1.67 × 1020 at 25°C for the formation of iron(III) oxalate complex ion: Fe3+(aq) + 3 C2O42-(aq) ⇌ [Fe(C2O4) 3]3-(aq) . If 0.0200 M Fe3+ is initially mixed with 1.00 M oxalate ion,what is the concentration of Fe3+ ion at equilibrium?
A) 1) 44 × 10-22 M
B) 0) 0100 M
C) 8) 35 × 1019 M
D) 6) 94 × 1021 M
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent the equilibrium state for four different reactions of the type
A2 + X2 ⇌ 2 AX (X = B,C,D,E) .A atoms are unshaded.X atoms are shaded. 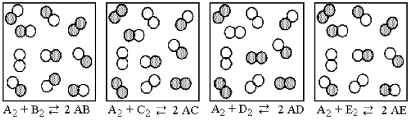 -Which reaction has the smallest equilibrium constant?
-Which reaction has the smallest equilibrium constant?
A) A2 + B2 ⇌ 2 AB
B) A2 + C2 ⇌ 2 AC
C) A2 + D2 ⇌ 2 AD
D) A2 + E2 ⇌ 2 AE
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
For the reaction shown below the value of Kp is ________ than the value of Kc,because Δn = ________. N2O4(g)⇌ 2 NO2(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which equilibrium below is homogeneous?
A) BaSO4(s) ⇌ Ba2+(aq) + SO42-(aq)
B) 2 H2O2(l) ⇌ 2 H2O(l) + O2(g)
C) NH4NO3(s) ⇌ N2O(g) + 2 H2O(g)
D) 2 CO(g) + O2(g) ⇌ 2 CO2(g)
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction reaches dynamic equilibrium at a given temperature when
A) the amount of products exceeds the amount of reactants.
B) kfwd equals krev.
C) opposing reactions cease and the system is static.
D) the relative amounts of reactants and products are constant and ratefwd = raterev.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If Kc = 2.0 x 1033 at 25°C,for the following reaction: H2(g) + Cl2(g) ⇌ 2 HCl(g) ,then find Kp at the same temperature.
A) 8) 2 × 1031
B) 9) 7 × 1032
C) 2) 0 × 1033
D) 4) 9 × 1034
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
The reaction CaCO3(s)⇌ CaO(s)+ O2(g)is endothermic 298 K.The effect of increasing the temperature of the system at equilibrium will ________ (decrease,increase,have no effect on)the total quantity of CaCO3 once equilibrium is reestablished.
Correct Answer

verified
Correct Answer
verified
Short Answer
The reaction CaCO3(s)⇌ CaO(s)+ O2(g)is endothermic 298 K.The effect of adding additional CaO to the system at equilibrium will ________ (decrease,increase,have no effect on)the total quantity of CaCO3 once equilibrium is reestablished.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium constant Kc for the reaction HF(aq) + H2O(l) ⇌ H3O+(aq) +F-(aq) is 3.5 × 10-4.What is the equilibrium concentration of H3O+ if the initial concentration of HF is 1.0 M?
A) 1) 0 M
B) 3) 5 × 10-2 M
C) 1) 9 × 10-2 M
D) 1) 9 × 10-4 M
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the equilibrium equation for the reverse reaction: 2 CH4 (g) + 3 O2 (g) ⇌ 2 CO (g) + 4 H2O (g)
A) Kc´ = ![]()
B) Kc´ = ![]()
C) Kc´ = ![]()
D) Kc´ = ![]()
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the reaction: 2 HI ⇌ H2 + I2.If Kc' for the reverse reaction is 1.85 × 10-2 at 425°C,what is Kc for the forward reaction at the same temperature?
A) -1.85 × 10-2
B) 1) 85 × 10-2
C) 3) 70 × 10-2
D) 54.1
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the equilibrium equation for the reverse reaction: 2 CH4(g) + 3 O2(g) ⇌ 2 CO(g) + 4 H2O(g)
A) Kp' = ![]()
B) Kp' = ![]()
C) Kp' = ![]()
D) Kp' = ![]()
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pink and blue species below form a violet colored mixture at equilibrium: [Co(H2O) 6]2+ (aq) + 4 Cl- (aq) ⇌ [CoCl4]2- (aq) + 6 H2O (l) (pink) (blue) If the concentration of [Co(H2O) 6]2+ is increased,what happens to the solution?
A) The concentration of [CoCl4]2- increases.
B) The concentration of [CoCl4]2- decreases.
C) The solution becomes colorless.
D) No color change is observed.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
A reaction in which reactants form products in the forward reaction and products simultaneously form reactants in the reverse reaction is said to be ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If Kc is the equilibrium constant for a forward reaction,2 A⇌ B,what is Kc´ for the reaction 4 A⇌ 2B?
A) ![]() Kc
Kc
B) Kc
C) 2 Kc
D) (Kc) 2
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phosphorus pentachloride decomposes to phosphorus trichloride at high temperatures according to the reaction: PCl5(g) ⇌ PCl3(g) + Cl2(g) At 250°C,0.250 M PCl5 is added to a flask.If Kc = 1.80,what are the equilibrium concentrations of each gas?
A) [PCl5] = 0.0280 M,[PCl3] = 0.222 M,[Cl2] = 0.222 M
B) [PCl5] = 1.25 M,[PCl3] = 0.474 M,[Cl2] = 0.474 M
C) [PCl5] = 1.80 M,[PCl3] = 1.80 M,[Cl2] = 1.80 M
D) [PCl5] = 2.27 M,[PCl3] = 2.02 M,[Cl2] = 2.02 M
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 166
Related Exams